Let’s talk about sex: Exploring evolutionary explanations for the persistence of sexual reproduction in eukaryotes
Published · Updated

A review by Mads S. Michalsen
1. Introduction
Why sex? Perhaps the simplest, yet deepest, question about sexual reproduction is why it exists at all. The answer seems obvious at first, to produce offspring. On second thought however, the question becomes far more intriguing. Because, as August Weismann said all the way back in 1889: "The significance of amphymixis (i.e. sex) cannot be that of making multiplication possible, for multiplication may be effected without amphimixis in the most diverse ways" (Weismann, 1889). In other words, sex is not the only option for reproduction. In eukaryotes, asexual modes of reproduction include budding, vegetative reproduction and parthenogenesis (Futuyma and Kirkpatrick, 2017). Furthermore, asexual modes of reproduction have advantages that should make them far more favorable than sex.
For one, asexual reproduction avoids the costs that come with finding a mate, including costs associated with sexual selection dynamics. The dangers of contracting sexually transmitted diseases and other risks connected to the act of mating are other costs not incurred. Additionally, sexual reproduction systematically breaks down successful genetic combinations through recombination and outcrossing. By far the most significant cost to sex is however what John Maynard-Smith coined the two-fold cost of males. Maynard-Smith (1971) argued that mutations that cause an organism to reproduce asexually should rapidly dominate if it arrived in a (dioecious) sexual population. Because asexual females do not invest in sons, the reasoning is that their birth rate should far exceed that of their sexual counterparts. Holding all else equal between sexual and asexual females, the cost of sex is expected to be two‐fold in outcrossing populations with separate sexes and equal sex ratios (Gibson et al., 2017). The two-fold cost also manifests itself in gene numbers. Each gamete only passes on 50 percent of its parent's genes [1]Interestingly, this opens the door for yet another cost to sex; the propagation of selfish genetic parasites (see e.g., Lane (2010)). If an individual were to attain a way to pass 100 percent of its genes to the next generation, e.g. by cloning, it would gain a two-fold advantage over the other individuals in the population. Its genes should soon dominate the population.
Considering all these costs, sex as a successful mode of reproduction seems paradoxical. It suffers a two-fold cost compared to asexual reproduction, it comes with the burden of finding and attracting a mate, it can transmit venereal diseases and it systematically breaks down successful genetic combinations.
Despite all of this, sex is ubiquitous among complex life forms. Almost all eukaryotes reproduce sexually at some stage of their life cycle, and many are obligate sexual reproducers. Only about 1 percent of plants and 0.1 percent of animal species reproduce asexually, i.e. by making genetic clones of themselves (Otto, 2009). Furthermore, most species with obligate asexual reproduction are recently formed, implying that fully asexually reproducing organisms generally have a short species-level life span in evolutionary time [2]A notable exemption from this is bdelloid rotifers, as this group of planktonic organisms have maintained an asexual reproductive mode for more than 100 million years (Fontaneto et al., 2007). The fact that most eukaryotes reproduce sexually is evidence of its evolutionary success, which entails that there must be major advantages to this form of reproduction. But what are they? Essentially, why does sex exist?
The question of why sex exists comprises two related, yet separate, topics: the origin of sex and its persistence through evolutionary time [3]For a discussion on the origin of sex, see e.g. Cavalier-Smith (2002). This paper limits itself to the latter, reviewing some of the different hypotheses put forth for the evolutionary advantages of sexual reproduction, in an attempt to make sense of its enduring prevalence despite its great costs.
2. Discussion
Initially, some quick definitions are in order. Reproduction is the process by which organisms give rise to offspring. Sexual reproduction can be defined as when a new organism is produced by the mixing of the genomes of two organisms of different types (parents) (Reece et al., 2014). Both parents produce gametes through meiosis, during which the chromosomes undergo recombination where genetic information is exchanged between the homologous chromosomes. Through this process every individual gamete produced by an organism are genetically unique to each other. Through outcrossing and the fusion of sex cells, 50 percent of the genetic material from each parent is combined in a zygote. This results in offspring that are genetically different from each other and from their parents. In short, sex is recombination and outcrossing, and the mixture of genes can be considered its primary feature. It does so systematically across the entire genome, breaking down previous combinations and generating new ones. But why?
2.1 The Vicar of Bray and Selective Interference
An early answer to the question of why sex persists dates back to the 19th century and the previously mentioned August Weismann. He suggested that the key benefit of sex is that it generates greater variation for natural selection to act upon, claiming it as "the source of individual variability, furnishing material for the operation of natural selection" (Weismann, 1889). Weismann further argued that sex was just as likely to generate detrimental as beneficial combination of genes, meaning that there is no net advantage to sex for the individuals in any given generation. The population as a whole, however, does benefit, as detrimental combinations are weeded out of the population by means of natural selection, leaving the favorable combinations. Bell (1982) later dubbed Weismann's view on the evolutionary purpose of sex the Vicar of Bray hypothesis. Closely linked to this is the idea that sex reduces selective interference, separating alleles from their genomic backgrounds allowing for more efficient adaptive selection processes (Futuyma and Kirkpatrick, 2017).
It might be important to emphasize that sex does not itself introduce new variation to a population in terms of new genes or alleles. Mutation (or gene flow) is needed for this. Without this, sex only generates new combinations of existing genes, eliminating disadvantageous variants along the way. Without the introduction of new variants, sex actually restricts variation.
After Weismann, a continuation of the Vicar of Bray hypothesis was formulated in terms of population genetics by Fisher (1930) and Muller (1932), among other things incorporating the role of mutations in a clearer way.
Figure 1 illustrates the resulting Fischer-Muller model, which demonstrates an advantage to sexual reproduction through the avoidance of clonal interference. Consider the outcome in an asexually reproducing population if advantageous mutations A and B appear (at different loci) in different individuals. Due to the mutations being beneficial, the clones of these individuals proliferate. Becoming common in the population, the two clonal lines start to compete, potentially driving one of the lineages to extinction (in this case aB). The only way for an AB genotype to get fixed in the population, is for both mutations to independently arise in the same clonal lineage, an unlikely event. In short, clonal interference is a hindrance to adaptive evolution in the population.
For a sexually reproducing population on the other hand, novel advantageous genotypes can be created more rapidly. If allele A and B arise independently in different individuals, the two alleles can rapidly be recombined in the same organism. Sexual reproduction allows the beneficial AB genotype to quickly spread to fixation.
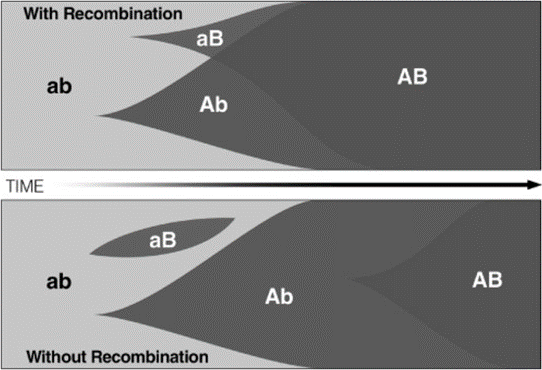
Figure 1: The spread of advantageous mutations in a sexual (top) and an asexual population (bottom). In the sexually reproducing population, the advantageous mutations (A and B) are quickly combined and the AB genotype brought to fixation due to recombination and selection. Without sex however, A can only spread at the expense of B and vice versa, unless both mutations arise in the same clonal lineage.
Crow and Kimura (1965) later provided a modern demonstration of the Fischer-Muller model. Through mathematical formulations they demonstrated the relative rates of incorporation of the new mutations with and without recombination. They found that recombination is of the greatest advantage when the double mutant is more advantageous than either single mutant, when the mutant effects are small, when mutations occur with high frequency, and when the population is large.
The Fisher-Muller model presents an argument for how sex can be beneficial through eliminating competition among favorable mutations that have arisen in different genetic backgrounds, instead bringing these together through recombination, speeding up adaptation. Later, Muller shifted focus from favorable mutations to deleterious ones, perhaps inspired by the fact that most non-neutral mutations are in fact deleterious (Loewe and Hill, 2010).
Muller (1964) introduced the concept later dubbed Muller's ratchet, a process in which, in the absence of recombination, deleterious mutations accumulate in a population in an irreversible manner. Without recombination, assuming that back-mutations are rare, Muller argues that offspring will at minimum carry the same mutational load as their progenitors. He proposed this mechanism as a further reason for why sex might win out over asexual reproduction, as sexually reproducing organisms avoid the ratchet due to sex's ability to recreate high-fitness individuals by bringing together unmutated alleles in the same individual. Experiments have shown that ratchet is fastest, and thus the advantage of sex the largest, when mutation rates are high, selection is weak, the organisms (genomes) are large and the size of the population is small (Bell and Graham, 1988).
Kondrashov (1988) complements the work of Muller by introducing the role of synergistic epistasis. This is the idea that most mutations are only slightly deleterious individually but that the cumulative effect of mutations has an increasingly large effect on fitness as the total number of mutations increases (see Figure 2). In a sexual population, some of the individuals born will have many mutations, while some will have few. Since there is a major selective disadvantage to individuals with more mutations, these individuals die out. Sex compartmentalizes the deleterious mutations, and rids the population of them.
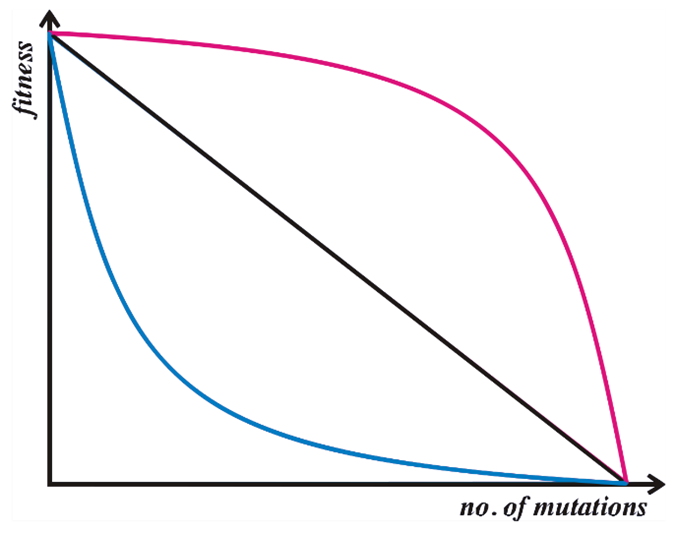
Figure 2: Illustration of different relationships between the number of mutations and fitness. The red line represents synergistic epistasis, where each subsequent mutation has a disproportionately large effect on the organism's fitness. This is a requirement for Kondrashov's model. The other two lines illustrate additive (black) and antagonistic (blue) effects of the number of mutations on fitness (Kondrashov, 1988). Original illustration by MyvReeve, licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license
The ideas presented so far seem to argue that sex persists because it benefits populations by bringing advantageous combinations of genes together, and by eliminating deleterious ones. Mystery solved? Many would argue no. One reason for this is that major parts of the Vicar of Bray thinking can essentially be deemed group selectionist, due to many of the benefits put forth mostly accruing to the group, and not the individual (at least not in any immediate sense). Given that there is a strong consensus that natural selection works on the level of the individual (or even the gene), and not on the species or group level, this poses a problem. Natural selection is not going to promote genes that benefit the species but hurt the individual, because such genes will be outcompeted by non-self-sacrificial variants (Maynard-Smith, 1978). Furthermore, Muller’s ratchet, the Fischer-Muller model, and other forms of reducing selective interference are slow processes. Their benefits do not seem to counter the large costs of sexual reproduction in the short term (Ridley, 1994). Convincing arguments for how sexual individuals can be better off than non-sexual individuals are therefore needed.
2.2 The Ecological Perspective
In the attempt to answer how sex sufficiently benefits the individual despite its two-fold cost, many biologists shifted focus towards ecological explanations. Williams (1975) argued that the genetic mixing inherent to sex, might prove beneficial when the environment is changing rapidly. If the environment changes from generation to generation, the genotype advantageous in one generation might not be advantageous in the next. The much greater diversity of sexually produced offspring compared to asexual ones, could therefore be a good strategy. However, if sex' purpose mainly were as an adaptation to highly variable environments, one would predict sex to be more prevalent in highly fluctuating environments such as high latitudes and altitudes. Generally speaking, this is not what is found. In fact, data suggest sex is actually more common in stable environments such as lakes and tropical rainforests (Bell, 1982). Williams' theory is not without value, but it seems to fall short as a single explanation for the persistence of sex.
Another ecological argument for sex is the Tangled Bank hypothesis. The Tangled Bank holds that since the state of the environment varies spatially even at fairly local levels, different genotypes will be optimal at different locations. Given that each location will only support a limited number of individuals, clonal offspring from an asexual parent will compete intensely with one another for the same set of resources. The offspring of a sexual female, however, by virtue of their greater diversity, will be able to exploit a much wider range of sites as well as different niches in the same location. This might lead to sexual lineages competing less amongst themselves and thus to greater overall success compared to asexual lineages despite its lower reproductive efficiency (Bell, 1982). An elegant idea, but empirical support for the Tangled Bank hypothesis as the driving cause for the persistence of sex is not very strong (Lane, 2010).
Lastly, a promising explanation of how sex can be favored by natural selection is referred to as the Red Queen hypothesis. This ecological hypothesis is named after a character in Lewis Carroll's Through the Looking Glass, who must "do all the running [she can do] in order to stay in the same place". Whilst the Red Queen term is used by biologist as a general reference to perpetual arms races between different species, it is perhaps most strongly associated with the evolution of sex [4]See e.g. Ridley (1994).
The hypothesis states that species are in evolutionary arms races with other species, and particularly parasites rapidly evolving to circumvent its hosts’ defenses. This produces a continually shifting adaptive landscape for the host organism, and in this context, sex can be a winning strategy. Recombination allows for the continual arrival of new combinations of alleles to which the parasite is not well-adapted. In the context of arms races, sexual reproduction and its mixing of genes can prove more advantageous than asexual reproduction and its slower production of novelty. Support for this can be found from the observation of parasite co-evolution in populations of mud snails (Potamopyrgus antipodarum), which have both sexual and asexual genotypes. Studying these populations, it was found that the populations with higher exposure to parasites shifted towards higher frequencies of the sexual genotype (Vergara et al., 2014). These studies, and others, suggest that the evolutionary benefits of the genetic mixing produced by sex can compensate for its costs.
The Red Queen hypothesis provides a strong argument for the primary purpose of sex being that of keeping parasites at bay. There are however doubts as to whether the threat of parasites is a strong enough force to explain the powerful pervasiveness of sex. For example, Howard and Lively (1994) used computer simulations to (among other things) study the role of parasites in explaining the rarity of obligate parthenogenesis in natural populations. They found that their models required that parasite transmission rates were very high, and that parasites had severe fitness effects on their hosts (>80% loss of fitness), in order for sex to gain a decisive advantage over parthenogenesis. Conditions like these are unquestionably present in a number of natural systems, but in no way all.
3. Synthesis
Many promising theories have been put forth in the quest to resolve the enigma of sex. It seems, however, that no single theory can provide strong enough explanatory power to alone account for the incredible persistence of sex. That being said, there is actually no reason why one theory alone should have to explain the evolutionary success of sexual reproduction. The theories reviewed in this paper are not mutually exclusive, so perhaps the explanation for sex lies in some combination of these ideas [5]Along with others not covered. See e.g. Bernstein et al. (1987) for an interesting argument for sex' primary value as a tool for DNA repair.
Considering Muller's ratchet and the Red Queen together, for example, does in fact provide a far stronger case for the advantage of sex over asex than if each factor is considered individually (Howard and Lively, 1994). Furthermore, more recent mathematical modelling work, such as that of Otto and Barton (1997) and Keightley and Otto (2006), have provided highly promising results through combining even more of the ideas discussed in this review. Through incorporating different mechanisms of selective interference in finite populations with the presence of strong selective forces such as parasites or rapidly changing environments into the same model, they are able to demonstrate that sex can immediately benefit the individual and perform better than cloning in a wide range of circumstances.
To conclude, sex reduces selective interference, bringing together favorable genetic combinations in the same individual and getting rid of deleterious mutations, and it arms the organism with the weapon of variability in the face of dynamic, adaptive landscapes. Sexual reproduction undeniably comes with great costs, but through a mixture of factors reminiscent of the process of sex itself, it prevails.
How to cite this article
Michalsen, M. S. (2021). Let’s talk about sex: Exploring evolutionary explanations for the persistence of sexual reproduction in eukaryotes. Bikuben 1.
Footnotes
[1] Interestingly, this opens the door for yet another cost to sex; the propagation of selfish genetic parasites (see e.g., Lane (2010))
[2] A notable exemption from this is bdelloid rotifers, as this group of planktonic organisms have maintained an asexual reproductive mode for more than 100 million years (Fontaneto et al., 2007)
[3] For a discussion on the origin of sex, see e.g. Cavalier-Smith (2002)
[4] See e.g. Ridley (1994)
[5] Along with others not covered. See e.g. Bernstein et al. (1987) for an interesting argument for sex' primary value as a tool for DNA repair
References
Bell, G. (1982), The masterpiece of nature: the evolution and genetics of sexuality, Routledge.
Bell, G. and Graham, B. (1988), Sex and death in Protozoa: the history of obsession, Cambridge University Press.
Bernstein, H., Hopf, F. A. and Michod, R. E. (1987), The molecular basis of the evolution of sex, in ‘Advances in Genetics’, Vol.24, Elsevier, pp.323–370.
Burden, A. (2017), Hornet eating flower, Unsplash
Cavalier-Smith, T. (2002), ‘Origins of the machinery of recombination and sex’, Heredity 88(2),125–141.
Crow, J. F. and Kimura, M. (1965), ‘Evolution in sexual and asexual populations’, The American Naturalist 99(909), 439–450.
Fisher, R. (1930), ‘The genetical theory of natural selection. clarendon’.
Fontaneto, D., Herniou, E. A., Boschetti, C., Caprioli, M., Melone, G., Ricci, C. and Barraclough, T. G. (2007), ‘Independently evolving species in asexual bdelloid rotifers’, PLoS biology 5(4).
Futuyma, D. and Kirkpatrick, M. (2017), Evolution, 4th edition, Sinauer.
Gibson, A. K., Delph, L. F. and Lively, C. M. (2017), ‘The two-fold cost of sex: experimental evidence from a natural system’, Evolution letters 1(1), 6–15.
Howard, R. S. and Lively, C. M. (1994), ‘Parasitism, mutation accumulation and the maintenance of sex’, Nature 367(6463), 554–557.
Keightley, P. D. and Otto, S. P. (2006), ‘Interference among deleterious mutations favours sex and recombination in finite populations’, Nature 443(7107), 89–92.
Kondrashov, A. S. (1988), ‘Deleterious mutations and the evolution of sexual reproduction’, Nature 336(6198), 435–440.
Lane, N. (2010), Life ascending: the ten great inventions of evolution, Profile books.
Loewe, L. and Hill, W. G. (2010), ‘The population genetics of mutations: good, bad and indifferent’.
Maynard-Smith, J. (1971), The origin and maintenance of sex, in ‘Group selection’, Routledge, pp. 163–175.
Maynard-Smith, J. (1978), The evolution of sex, Vol. 4, Cambridge University Press Cambridge.
Muller, H. J. (1932), ‘Some genetic aspects of sex’, The American Naturalist 66(703), 118–138.
Muller, H. J. (1964), ‘The relation of recombination to mutational advance’, Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 1(1), 2–9.
Otto, S. P. (2009), ‘The evolutionary enigma of sex’, The American Naturalist 174(S1), S1–S14.
Otto, S. P. and Barton, N. H. (1997), ‘The evolution of recombination: removing the limits to natural selection’, Genetics 147(2), 879–906.
Reece, J. B., Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., Jackson, B. et al. (2014), Campbell biology, number s1309, Pearson Boston, MA.
Ridley, M. (1994), The red queen: Sex and the evolution of human nature, Penguin UK.
Vergara, D., Jokela, J. and Lively, C. M. (2014), ‘Infection dynamics in coexisting sexual and asexual host populations: support for the red queen hypothesis’, The American Naturalist 184(S1), S22–S30.
Weismann, A. (1889), ‘The significance of sexual reproduction in the theory of natural selection’, Essays upon heredity and kindred biological problems 1, 255–332.
Williams, G. C. (1975), Sex and evolution, number 8, Princeton University Press.
About the author
 Author Mads. S Michalsen finished his bachelor’s degree in biology at UIB in 2019. He had already finished his studies in industrial engineering (NTNU) and took up Biology out of curiosity and personal interest for the field. Mads is now living in Oslo, where he works as a management consultant for PwC.
Author Mads. S Michalsen finished his bachelor’s degree in biology at UIB in 2019. He had already finished his studies in industrial engineering (NTNU) and took up Biology out of curiosity and personal interest for the field. Mads is now living in Oslo, where he works as a management consultant for PwC.
When we asked what motivated him to write about this subject, Mads answered:
The question of why sex (specifically gendered reproduction as a strategy) is prevalent, is a theme I wished to explore because it represents one of my favorite problems: the type where the answer at first seems obvious, but on closer look is a real mystery – in this case an evolutionary riddle. It is also fun to review a field which has a century old history, but where there still remains lively debate and no consensus has been reached. This was also a chance to exercise the practice of robust, evolutionary logic.

2 Responses
[…] “Let’s talk about sex: Exploring evolutionary explanations for the persistence of sexual reprodu… […]
[…] off, but are now slowly receiving more contributions. So far we have published two texts online: “Let’s talk about sex: Exploring evolutionary explanations for the persistence of sexual reproducti…” by Mads S. Michalsen, and “The Insect Apocalypse: a cause for concern or simply an […]