The Hidden Pollinators of Inner Hardanger: a study in Hoverfly (Syrphidae) Diversity, Abundance, and Phenology
Published · Updated

A report written by Sara Rodrigues de Miranda
Abstract
Hoverflies (Syrphidae) are a family of flies of the Diptera order that provide a range of ecological services to agriculture, including pollination services. Most crops are reliant on animal-plant interactions to aid their pollination, including those intended for human consumption such as apple trees. While Hymenopteran species are the most well-known insects that provide pollinating services, hoverflies are the second most important pollinators. However, hoverflies are often partly overlooked in scientific research, commonly only identified to family. Little is therefore known about the diversity, abundance, and phenology of this family of insects. This report will give an overview of what hoverflies are, their importance to agriculture, and subsequently what services they provide to aid pollination and ecosystems. A description of species diversity, abundance, and phenology in inner Hardanger will be provided. Additionally, this text will discuss i) the differences in hoverfly diversity of different sites in inner Hardanger, ii) what hoverfly diversity tells us about the ecological integrity of these sites, and iii) the phenology of hoverflies in Hardanger and what the differences in species phenology can tell us about their species-specific importance as apple flower pollinators in apple orchards in Hardanger. The data from this report has been collected in connection with the APPLECore project, specifically for Silje Maria M. Høydal´s master thesis on bee pollinators in apple orchards in Hardanger, Norway. Hoverflies were not the intended insects for capture in this project, and it is therefore noted that the methodology is not streamlined for the capture of hoverflies.
Introduction
Pollination is an ecological service that is essential to the success of agricultural practices and is provided by a range of different animals. Amongst animals that provide pollinating services, insects are the largest and most important group (Doyle et al., 2020). Pollinating insects are insects that aid in the fertilization of plants (Berner & Sunding, 2021). This can occur in differing ways, the most common being a visit to a flower by an insect foraging for nectar or pollen (Berner & Sunding, 2021). The insects most famous for pollinating services for fruit-bearing plants are insects of the Hymenoptera order and the flies of the Syrphidae family. Although many plants do not require insect-specific pollinating services, up to 76% of crops intended for human consumption are reliant on animal-crop interactions to aid the pollination process (Bates et al., 2011; Doyle et al., 2020). Agriculture is a fundamental part of food production and has been a part of the foundation of modern societies. For the region of Hardanger, the production of apples has been a cornerstone of their economy and history for hundreds of years (Thorsnæs, 2021).
There are several factors that contribute to effective fruit yield, an essential one being the success of pollination during the spring (Ramírez & Davenport, 2013). Fruit trees, including apple trees, are reliant on animal-plant interactions, in particular visitations of insects on their flowers to aid their pollination (Berner & Sunding, 2021; Ramírez & Davenport, 2013). One of the reasons why apple trees specifically need insect visitations is due to insects being effective cross-pollinators, on which apple trees are reliant (Berner & Sunding, 2021; Ramírez & Davenport, 2013). Cross-pollination is a process in which pollen is transferred between the main cultivar and a polliniser cultivar (Berner & Sunding, 2021; Ramírez & Davenport, 2013). Farmers have long practiced keeping colonies of pollinating insects in the vicinity of their crops to aid pollination (Delaney & Tarpy, 2008). Honeybees (Apis mellifera, Linnaeus, 1758) is a species of social bees which have been domesticated and is amongst other things used for pollination of commercial fruit trees (Delaney & Tarpy, 2008). However, recent research shows that the pollination services provided by wild pollinators is more effective than those provided by domesticated bees (Bates et al., 2011; Berner & Sunding, 2021; Doyle et al., 2020). Wild pollinator diversity and abundance is therefore important for the increase in crop yield in apple orchards (Bates et al., 2011; Delaney & Tarpy, 2008; Ramírez & Davenport, 2013).
The Diptera order is amongst the most important wild pollinators. In fact, by regularly visiting 72% of crops, it is the second most important pollinating wild insect (Boyle & Philogène, 1983; Doyle et al., 2020). Hoverflies (Syrphidae) are a family of the Diptera order, which represent 52% of crop visitations attributed to all flies, displaying the importance of this family for the pollination of apple trees (Doyle et al., 2020; Ottesen, 2021; Ramírez & Davenport, 2013). They are popularly known for their Batesian mimicry of the insect order Hymenoptera which includes bees, bumblebees, and wasps (McLean et al., 2019; Nottingham, 2000; Penney et al., 2014; Wilson et al., 2013). Hoverflies are abundant and diverse in Norway and are known to visit many of the same fruit plants as bees (including bumblebees) (Ball & Morris, 2015; Bengtson et al., 2022; Djellab et al., 2019). Furthermore, the larvae of the subfamilies Syrphinae and Eristalinae have other ways to contribute services to their ecosystems; Syrphinae larvae are a natural enemy of the crop pest’s aphids (Aphidoidea) and have showed to lower pest populations by 70%, whereas the saprophagous Eristalinae larvae are known to decompose dead plant matter (Ball & Morris, 2015; Djellab et al., 2019; Doyle et al., 2020). Because of the aforementioned, they are an asset to their surrounding environments throughout their entire life cycle (Ball & Morris, 2015; Djellab et al., 2019; Doyle et al., 2020).
As a highly migratory species, hoverflies will travel great lengths throughout the year, providing a large geographical spread of pollinating services that widen ecological gene pools significantly (Doyle et al., 2020; Wotton et al., 2019). Throughout their life cycle, hoverflies make use of a wide range of habitats, and it has been shown that they thrive in specific microhabitats (Ball & Morris, 2015; Bengtson et al., 2022; Gittings et al., 2006; Lucas, 2017). They often make use of various forms of faeces, composts heaps, tree bark and herbaceous plants for laying eggs (Ball & Morris, 2015; Bengtson et al., 2022). Adult hoverflies that will specialize in a single plant type are typically early season fliers (Lucas et al., 2018). Of species that fly during late summer months, only a small portion are generalists that will inhabit a range of different types of habitats (Lucas et al., 2018). As such, these insects are often uniquely suited to correlate species diversity with ecological diversity (Ball & Morris, 2015; Bengtson et al., 2022; Gittings et al., 2006). Because of the close connection between hoverflies and specific plant types, hoverfly diversity can thus directly tell us vital information about the ecological integrity of an ecosystem (Djellab et al., 2019). Hoverflies undergo full metamorphosis, and have four main life stages of egg, larvae, pupae, and adult (Ball & Morris, 2015; Bengtson et al., 2022). The lifespan of most adult hoverflies is about 35 days, and they seldom become active at temperatures below 15˚C (Ball & Morris, 2015; Bengtson et al., 2022). As conservation strategy, they will therefore spend up to nine weeks during winter months and three weeks in summer months in their pupal stage (Ball & Morris, 2015; Bengtson et al., 2022; Weems, 2000). Hoverflies visit flowers to feed on nectar and pollen which aids them in ovulary production (Doyle et al., 2020). Largely due to this, phenological activity for species have been found to peak during flowering seasons of plants (early April-late August), which usually coincides with the same seasons and locations as bees, bumblebees, and wasps (Ball & Morris, 2015; Bengtson et al., 2022; Djellab et al., 2019; Howarth et al., 2004). Therefore, it is generally possible to observe an approximate positive correlation between the appearance of hymenopteran species and their syrphid mimics (Howarth et al., 2004; Penney et al., 2014).
Pollinator diversity and species richness have been shown to follow an urbanisation gradient, where bee and hoverfly diversity was found to be most diverse and abundant in rural areas, as opposed to being the least diverse urban areas (Bates et al., 2011; Luder et al., 2018). As such, conserving rural areas with highly diverse insect populations is expected to also benefit and support their surrounding habitats (Bates et al., 2011). Applying knowledge about the intrinsic connection between insect diversity and plant diversity will allow us to help increase insect populations in urban areas (Bates et al., 2011). One could therefore pose the question as to why a family of insects that provide such a wide range of agricultural and ecological services are relatively unprioritized both in academia and the media. The pollinator crisis has been central in media during the last 20 years of discussions surrounding environmental conservation (Balfour et al., 2018). Due to the decline in pollinator populations, research pertaining to the study of the Hymenoptera order has increased dramatically. However, there is little research focused on hoverflies on the west coast of Norway, or indeed worldwide. Due to this, there is a gap in knowledge related to the diversity, abundance, and phenology of hoverflies in Norway, specifically to the hoverflies in inner Hardanger.
Purpose
The purpose of this study is to provide an overview and analysis of diversity, abundance, and phenology of the hoverflies (Syrphidae) in a selection of apple orchards located in inner Hardanger from data collected in connection with the APPLECore project. The APPLECore project is led by the Norwegian Institute for Nature Research (NINA) in collaboration with the Norwegian Institute of Bioeconomy Research (NIBIO), where they are investigating a series of questions surrounding pollination and ecology (APPLECore, 2021). The effect of pollinator diversity in apple orchards on autumnal fruit yield is among the research questions of the APPLECore project (APPLECore, 2021).
Material & methods
Choice of data analysis
The data analyses for this study included: i) Shannon-Weaver diversity index and ii) species accumulation plots.
The Shannon-Weaver diversity index was used as Alpha Diversity estimate used for the evaluation of species diversity for the three sites. Alpha diversity is an evaluation of how diverse a sample is (Willis, 2019). The Shannon-Weaver diversity index gives a numeric estimate of species diversity and richness for a location (Bobbitt, 2021; Ortiz-Burgos, 2016). The formula for calculating Shannon-Weaver diversity index is:
![]()
where:
pi = proportion of each species in sample
log2pi = natural logarithm of pi proportion
Shannon-Weaver diversity index gives a value between 0-4.5, where the usual values range between 1.5-3.5 (Bobbitt, 2021; Ortiz-Burgos, 2016).
Species accumulation curves were used to estimate species diversity and richness and is a representation of sampling effectiveness for a site (Deng et al., 2015). If the curve of a species accumulation plot is flattened, the samples analysed are representative of the whole species diversity for a site (Deng et al., 2015). If a species accumulation plot is still rising, the data analysed is an underrepresentation of site-specific species diversity, and further data is required to accurately represent all species that are discoverable (Deng et al., 2015). The shaded area of a species accumulation curve shows a 95% confidence interval (Deng et al., 2015).
Sampling design
Triplets of pan traps (yellow, blue, and white) and blue vane traps were placed in the locations Djønno, Urheim and Opedal in Hardanger, Western Norway between late April – mid-June 2022. Djønno and Urheim are located in relatively rural areas, whereas Opedal is located in a more urbanized area. Six triplets of pan traps and three blue vane traps were placed in each of the three locations (Høydal, 2022). Both types of traps contained a soap water mixture to break the surface tension of the water to ensure insects drowned when caught (Høydal, 2022). The insects were sampled through four sampling periods separated by 14 days to prevent over trapping too early in the season (Høydal, 2022). In every sampling period the traps were placed in the field for four days and only emptied every two to three days (due to limitations in available workforce) (Høydal, 2022). Trap contents were bagged and labelled and stored in a freezer prior to identification.
In the laboratory, insects were defrosted and all insects with a false wing vein Vena spuria were selected. After hoverflies were identified to species, they were pinned through thorax with a size 000 pin needle. Individuals identified were given a species_ID. Every individuals’ identification was double checked using materials provided by The University Museum of Bergen, of which included Swedish identification literature and pinned museum examples of the most abundant species identified (Bartsch, 2009b; Bartsch, 2009a; Gammelmo, 2017).
Data Analysis
Data summarised in the excel sheet was exported and loaded into an R Studio workspace where the software R version 4.0.3 was used to process the data (The R Foundation, 2020). To visualise species diversity and abundance, phenological activity and trap colour and trap preference we used the plot() and barplot() functions. The values of Shannon-Weaver diversity index for each of the three sites were calculated and were used as a numerical estimation of diversity. Shannon-Weaver diversity index was calculated using the diversity function, diversity() from the vegan package version 2.5-7 (Oksanen et al., 2022). To check if the sampling portion was an adequate representation of species in the areas, we produced a species accumulation curve. The species accumulation curve was produced by firstly collecting data for species and all traps as a value using the table() function. Secondly, using the default extract method for the specaccum() function on the values sorted into a table, a polygon plot was made by using the plot() function with the specification of ci.type being “polygon”.
Results
Phenological activity
Hoverflies were found to be mostly active during the summer months of June (Figure 1). The highest abundance of hoverflies was found to be mid-June in post-flowering period of apple trees (Figure 1).
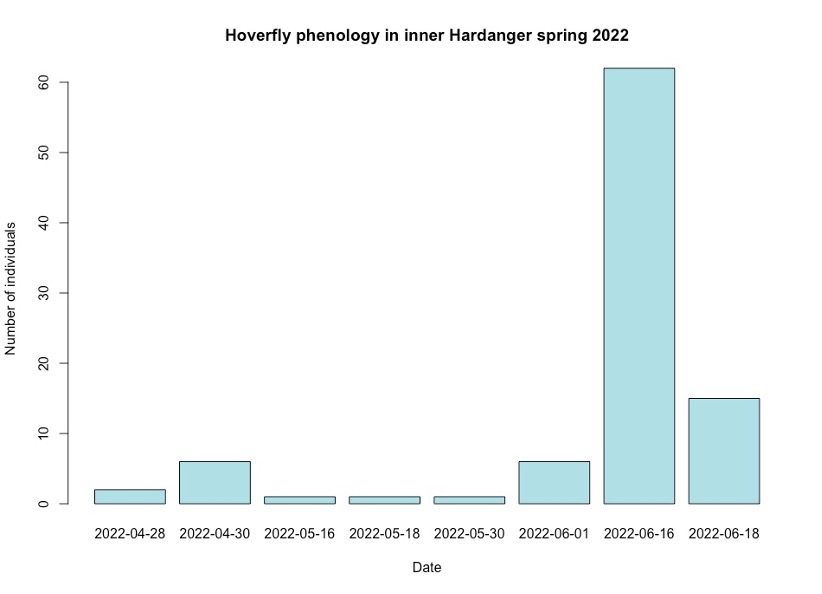
Figure 1: Bar graph showing hoverflies caught on dates throughout the field season in total for all locations.
Shannon-Weaver diversity index
The Shannon-Weaver diversity index for Urheim was approximately 2.66 and was the most diverse site. Close to Urheim on the diversity index was Djønno with an approximate value of 2.52. The least diverse site with an approximate value of 1.71 was Opedal.
Species accumulation curve
The species accumulation curve did not flatten (Figure 2). Due to the rising graph, individuals of the hoverfly family Syrphidae collected was likely an underrepresentation of the available hoverflies in Djønno, Urheim and Opedal (Figure 2). The species accumulation curve did not represent the total species richness for inner Hardanger (Figure 2).
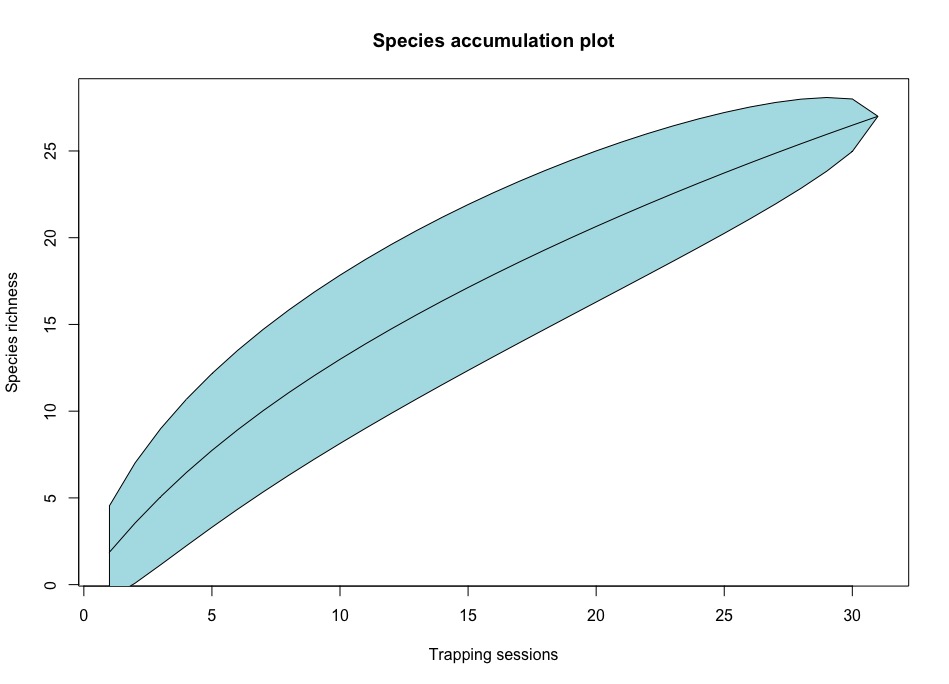
Figure 2: Species accumulation curve for hoverflies caught in all trap types throughout the entire field season.
Species abundance
The most abundant subfamily was found to be Eristalinae, representing 65.96% of all the individuals caught (Figure 3). Syrphinae represents the remaining 34.04% of the individuals caught (Figure 3). There were no individuals caught in the Microdontinae subfamily, and it is therefore not represented in this sampling.
Cheilosia spp. were found to be the most abundant hoverflies, representing 26.6% of all individuals collected. Collectively, Xylota spp. was nearly as abundant as Cheilosia spp., with a representation of 22.34% of individuals.
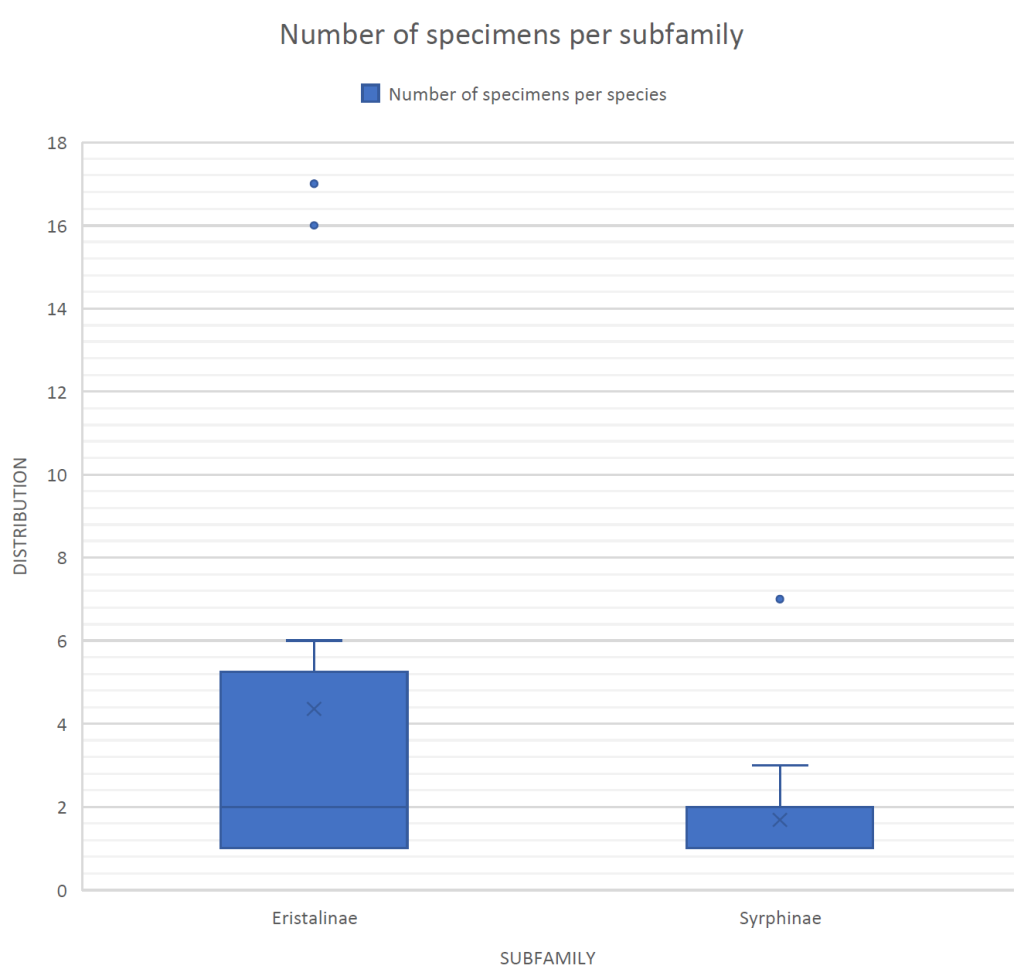
Figure 3: Boxplot displaying the distribution of specimen found for subfamilies Eristalinae and Syrphinae. No data for subfamily Microdontinae.
Site-specific Species abundance
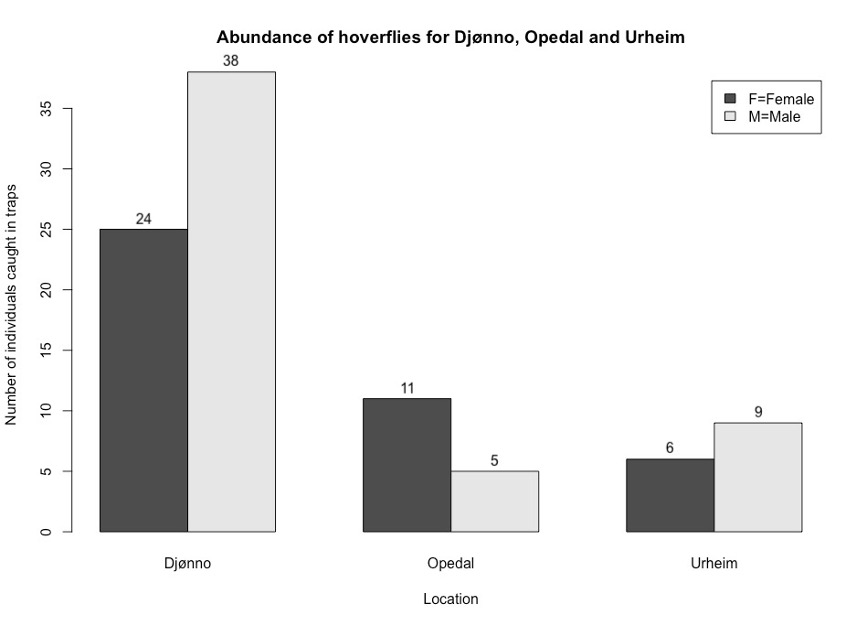
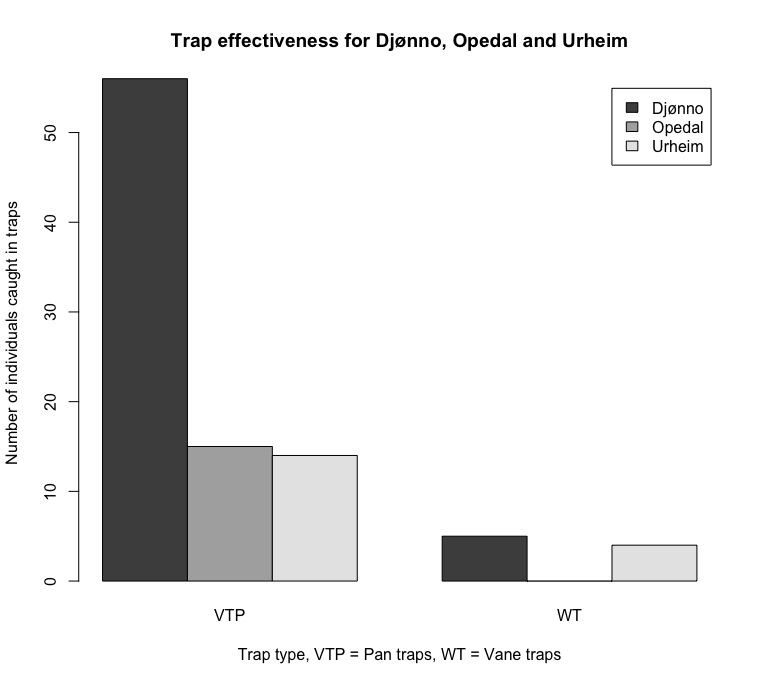
The highest abundances were found in Djønno, with 63 individuals (25 females, 38 males) (Figure 4). Urheim and Opedal were both similar in terms of hoverfly abundance (Figure 4). Identified from Urheim were 15 individuals (6 females, 9 males) (Figure 4). From Opedal, 16 individuals (11 females and 5 males) were identified (Figure 4). A disclaimer is placed here to inform that there were dates with missing trap materials for both Djønno and Opedal.
Methodological effectiveness
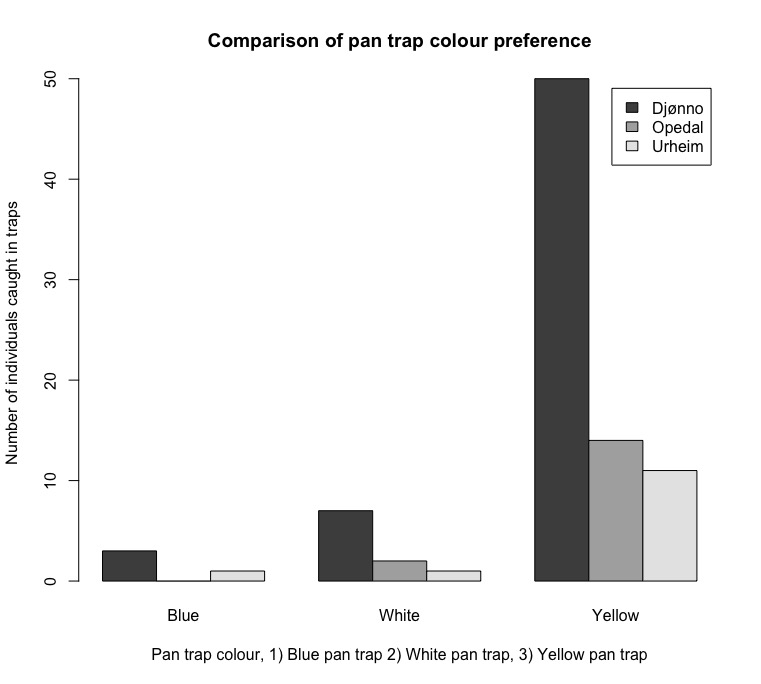
Insects collected using pan traps and vane traps under-sampled the available species for all sites (Figure 5). Pan traps were more effective than vane traps (Figure 5). Yellow pan traps were more effective than white and blue pan traps (Figure 6). 94 individuals of the hoverfly family were identified from this field season.
Discussion
Phenological activity
The results show that hoverflies in Hardanger become active at the same time as the beginning of apple tree flowering season, where they peak in activity in summer during mid-June (Figure 1)(Djellab et al., 2019). A peak around June is consistent with both optimal temperature levels for activity and hoverflies need for pollen during ovulary production (Figure 1)(Djellab et al., 2019). As their life span lasts about 35 days, the peak in activity in June would be expected and is consistent with the results (Figure 1). As the availability of pollen rises over time, a correlated abundance of hoverflies can be observed (Figure 1).
To adequately provide a true representation of the phenological activity, more consistent sampling would be needed. The 14-day period between trapping sessions potentially allowed for hoverfly species that are active between these periods to go undetected. In addition, one must also consider a different methodology. Sampling with an entomological net every day would be an alternative method that would allow for a line-graph to be produced and would likely yield a higher degree of certainty about the activity of hoverfly species (Bates et al., 2011; Gittings et al., 2006; van Steenis, 2016).
Shannon-Weaver diversity index
The hoverflies were found to be most diverse in Urheim and the most abundant in Djønno, the two most rural of the three sites. This is consistent with findings of earlier studies of pollinator diversity under urbanisation gradients (Bates et al., 2011). All Shannon-Weaver diversity index values are consistent with the estimated Hymenoptera order diversity for the sites as well. Additionally, diversity metrics for all three sites also corroborate with their urbanisation gradients (Bates et al., 2011). Opedal being a relatively urban site, roads and the cropland nearby are cultivated and relatively monocultured both in diversity of insects and plants. The diversity of Djønno was equally consistent with urbanisation gradients, as the site was far away from heavily trafficked roads, and the surrounding ecology was not as heavily modified from natural habitats. Urheim has median value with an index of 2.66, which corresponds with the site being in a relatively rural area. While the Shannon-Weaver diversity value found for Djønno was not as high as Urheim, the difference between the two sites is only a numerical value of 0.14, and as such, they are approximately as diverse. Nevertheless, there is a clear numerical division in the diversity index between the most urbanised site (Opedal) and the most rural sites (Urheim and Djønno).
Species accumulation curve
The species accumulation curve is still rising and shows no flattening (Figure 4). We can therefore ascertain that the data was an underrepresentation of the available hoverfly species diversity and richness for Djønno, Urheim and Opedal. Methods used for capture of insects are in a higher degree streamlined for the capture of Hymenoptera. To get a sample size that is properly representative for the area, one would need to be consider alternative methods for hoverfly capture, such as entomological netting.
The period where the traps where placed was broken into four periods. To obtain a greater representation of what the phenological activity of hoverflies looks like, one would need to have a consistent and continued sampling of all three areas. A greater sample size over a longer time period would therefore likely show a better representation of the diversity, abundance, and phenology of hoverflies in the area. Common methods for hoverfly capture used in studies of hoverflies are entomological nets or Malaise traps. Capture is usually done consistently at the same times and locations every day over a time period of between 5-12 months, with variations depending on the study (Bates et al., 2011; Gittings et al., 2006; van Steenis, 2016). Recognized here is that the main purpose of the trapping of insects was not to trap hoverflies, but rather to sample bees and bumblebees in the area.
Species diversity and abundance
All species identified were registered as having strong populations (abundant) in Norway (Artskart, 2022). Generally, hoverflies become active at around the same temperatures as the start of the flowering season of apple trees (~15˚C) (Bengtson et al., 2022; Ramírez and Davenport, 2013). In regard to the Hardanger region, a wide range of syrphids can be observed. The different species become active at different times during the year. Finding the species Melangyna barbifrons (Fallén, 1817), was particularly interesting, as this is a species of hoverfly that becomes active early in the year. The timing of Melangyna barbifrons activity coincides with the beginning of the flowering season of apple trees, and this species is therefore likely especially important for the pollination of apple trees (Ramírez and Davenport, 2013). There are no earlier registrations of this species in this area, and this species is therefore a particularly important find for Djønno (Artskart, 2022).
Other species found to be important for pollination are species of Eristalis spp. which have shown to carry similar pollen loads to Apis mellifera (Ramírez and Davenport, 2013). It is positive that an individual of Eristalis pertinax (Scopoli, 1763) was identified, where the region of Hardanger already have seven sightings recorded (Artskart, 2022). Uncommon species that were observed and not previously registered in Hardanger but are represented in our dataset include species such as Cheilosia proxima (Zetterstedt, 1843), Cheilosia pegana (Meigen, 1822), Chrysotoxum fasciolatum (De Geer, 1776), Orthonevna geniculate (Meigen, 1830) and Parasyrphus nigritarsis (Zetterstedt, 1843). Of hoverflies identified through this study, the species that have previously been found to be the most abundant in inner Hardanger area are the species Cheilosia sahlbergi (Becker, 1894), Platycheirus albimanus (Fabricius, 1781), Syritta pipiens (Linnaeus, 1758), Syrphus ribesii (Linnaeus, 1758), Syrphus vitripennis (Meigen, 1822) and Syrphus torvus (Osten-Sacken, 1875). These species are therefore expected to see in our dataset (Artskart, 2022). Notable mentions are species such as Ferdinandea cuprea (Scopoli, 1763), Cheilosia albitarsis (Meigen, 1822), and Xylota jakutorum (Bagathanova, 1980), which are species of which only a few sightings are recorded. They additionally represented some of the highest abundancies of specimens per species in our results (Artskart, 2022).
The results show a large variation of species that emerge and are active as adults at different parts of the year with a mix of species that are both early season fliers such as Melangyna barbifrons and late season fliers such as Melanostoma mellinum (Linnaeus, 1758), Syritta pipiens and Xylota segnis (Linnaeus, 1758) all of which are saprophagous late spring-early autumn fliers. There are species of hoverflies identified in this study that one would expect to see, such as Syritta pipiens which is a species known to inhabit compost heaps and a species that we could expect to find in areas that require farmers to use fertilizers on their crops (Bengtson et al., 2022). Melanostoma mellinum are species known to inhabit areas that include grasslands as they are a species that feed on grass pollen (Artsdatabanken, n.d.).
Species activity and abundance are both variables that can differ between years, and seasonal activity is a variable that also depends not only on flowering plants, but also on temperature and weather conditions (van Steenis, 2016). As such, it is recognized that there is a limitation to the dataset that is used in this study, and to be able to ascertain to a greater degree what species are expected in inner Hardanger, we would need to study these insects over a multitude of years. Hoverflies are likely a species that are underrepresented in The Norwegian database for species Artsdatabanken. Attention is drawn to this due to some species were found to be highly abundant in this study. Examples of such species are Cheilosia albitarsis and Xylota jakutorum which are rarely reported for the Hardanger area in the species register (Artskart, 2022).
Conclusion
Given the materials sampled, the peak phenological activity of hoverflies in inner Hardanger region was mid-June. Pan traps sampled hoverflies in greater degree than vane traps. Shannon-Weaver diversity metric showed that the rural areas of Djønno and Urheim were the most diverse, while Opedal, the most urbanised area, was the least diverse. Due to the missing dates and trap materials in data, there is uncertainty in the Shannon-Weaver diversity index for both Opedal and Djønno as opposed to Urheim. Species accumulation curve showed that traps underrepresented the available species, and the data collected is therefore unlikely a true representation of what the hoverfly community looks like. To obtain a better representation of diversity, abundance, and phenology of hoverflies in Hardanger, we would need to reassess the methodology used for capture and sample insects over a greater and more consistent time period.
How to cite
Rodrigues de Miranda, S. (2023). The Hidden Pollinators of Inner Hardanger: a study in Hoverfly (Syrphidae) Diversity, Abundance, and Phenology. Bikuben 2.
References
APPLECore. (2021). Full Proposal APPLECore. Norwegian Institute for Nature Research (NINA).
Artsdatabanken. (n.d.). Melanostoma mellinum. Retrieved December 2, 2022, from https://www.artsdatabanken.no/taxon/Melanostoma%20mellinum/22966
Artskart. (2022). [Databank]. Artsdatabanken. https://artskart.artsdatabanken.no/app/#map/43159,6689661/7/background/greyMap/filter/%7B%22IncludeSubTaxonIds%22%3Atrue%2C%22Found%22%3A%5B2%5D%2C%22NotRecovered%22%3A%5B2%5D%2C%22Style%22%3A1%7D
Ball, S., & Morris, R. (2015). Britans´s Hoverflies: A field guide (2nd ed.). Princeton University Press.
Balfour, N. J., Ollerton, J., Castellanos, M. C., & Ratnieks, F. L. W. (2018). British phenological records indicate high diversity and extinction rates among late-summer-flying pollinators. Biological Conservation, 222, 278-283. https://doi.org/10.1016/j.biocon.2018.04.028.
Bartsch, H. (2009a). Tvåvingar: Blomflugor, Diptera: Syrphidae: Eristalinae & Microdontinae. Nationalnyckeln til Sveriges flora och fauna.
Bartsch, H. (2009b). Tvåvingar: Blomflugor: Diptera: Syrphidae: Syrphinae. Nationalnyckeln til Sveriges flora och fauna.
Bates, A. J., Sadler, J. P., Fairbrass, A. J., Falk, S. J., Hale, J. D., & Matthews, T. J. (2011). Changing Bee and Hoverfly Pollinator Assemblages along an Urban-Rural Gradient. PLoS ONE, 6(8), e23459. https://doi.org/10.1371/journal.pone.0023459
Bengtson, R., Strømmen, F., & Østerhagen, E. (2022). Blomsterfluer- elegante, spennende og viktige. Statsforvalteren i Oslo Og Viken, 21.
Berner, E. jr., & Sunding, P. (2021). Pollinering – botanikk. In Store norske leksikon. http://snl.no/pollinering_-_botanikk
Bobbitt, Z. (2021, March 29). Shannon Diversity Index: Definition & Example. Statology. https://www.statology.org/shannon-diversity-index/
Boyle, R. M. D., & Philogène, B. J. R. (1983). The native pollinators of an apple orchard: Variations and significance. Journal of Horticultural Science, 58(3), 355–363. https://doi.org/10.1080/00221589.1983.11515130
Delaney, D. A., & Tarpy, D. R. (2008). The role of honey bees in apple pollination—Apiculture program, Department of Entomology. North Carolina State University. https://www.yumpu.com/en/document/read/15742378/the-role-of-honey-bees-in-apple-pollination-north-carolina-state-
Deng, C., Daley, T., & Smith, A. D. (2015). Applications of species accumulation curves in large-scale biological data analysis. Quantitative Biology (Beijing, China), 3(3), 135–144. https://doi.org/10.1007/s40484-015-0049-7
Djellab, S., Mebarkia, N., Neffar, S., & Chenchouni, H. (2019). Diversity and phenology of hoverflies (Diptera: Syrphidae) in pine forests (Pinus halepensis Miller) of Algeria. Journal of Asia-Pacific Entomology, 22(3), 766–777. https://doi.org/10.1016/j.aspen.2019.05.012
Doyle, T., Hawkes, W. L. S., Massy, R., Powney, G. D., Menz, M. H. M., & Wotton, K. R. (2020). Pollination by hoverflies in the Anthropocene. Proceedings of the Royal Society B: Biological Sciences, 287(1927), 20200508. https://doi.org/10.1098/rspb.2020.0508
Gammelmo, Ø. (2017). Sjekkliste for Blomsterfluer (Diptera, Syrphidae).
Gittings, T., O’Halloran, J., Kelly, T., & Giller, P. S. (2006). The contribution of open spaces to the maintenance of hoverfly (Diptera, Syrphidae) biodiversity in Irish plantation forests. Forest Ecology and Management, 237(1–3), 290–300. https://doi.org/10.1016/j.foreco.2006.09.052
Howarth, B., Edmunds, M., & Gilbert, F. (2004). Does the abundance of hoverfly (Syrphidae) mimics depend on the numbers of their hymnopteran models? Evolution, 58(2), 367–375. https://doi.org/10.1111/j.0014-3820.2004.tb01652.x
Høydal, S. M. M. (2022). Field Protocol Passive Sampling [Unpublished master thesis]. Department of Biological Sciences, University of Bergen.
Lucas, A. (2017). Hoverfly Communities in Semi-Natural Grasslands, and their Role in Pollination [PhD, Swansea University]. https://doi.org/10.23889/SUthesis.40892
Lucas, A., Bodger, O., Brosi, B. J., Ford, C. R., Forman, D. W., Greig, C., Hegarty, M., Neyland, P. J., & de Vere, N. (2018). Generalisation and specialisation in hoverfly (Syrphidae) grassland pollen transport networks revealed by DNA metabarcoding. Journal of Animal Ecology, 87(4), 1008–1021. https://doi.org/10.1111/1365-2656.12828
Luder, K., Knop, E., & Menz, M. H. M. (2018). Contrasting responses in community structure and phenology of migratory and non-migratory pollinators to urbanization. Diversity and Distributions, 24(7), 919–927. https://doi.org/10.1111/ddi.12735
McLean, D. J., Cassis, G., Kikuchi, D. W., Giribet, G., & Herberstein, M. E. (2019). Insincere Flattery? Understanding the Evolution of Imperfect Deceptive Mimicry. The Quarterly Review of Biology, 94(4), 395–415. https://doi.org/10.1086/706769
Nottingham, T. (2000). Mimicry and the hoverflies By Salma Azmeh , BSc ( Hons ).
Oksanen, J., Simpson, G. L., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’Hara, R. B., Solymos, P., H. Stevens, M. H., Szoecs, E., Wagner, H., Barbour, M., Bedward, M., Bolker, B., Borcard, D., Carvalho, G., Chirico, M., De Caceres, M., Durand, S., & Antoniazi Evangelista, H. B. (2022). Community Ecology Package. https://cran.r-project.org/web/packages/vegan/vegan.pdf
Ortiz-Burgos, S. (2016). Shannon-Weaver Diversity Index. In M. J. Kennish (Ed.), Encyclopedia of Estuaries (pp. 572–573). Springer Netherlands. https://doi.org/10.1007/978-94-017-8801-4_233
Ottesen, P. S. (2021). Blomsterfuer. In Store norske leksikon. http://snl.no/blomsterfluer
Penney, H. D., Hassall, C., Skevington, J. H., Lamborn, B., & Sherratt, T. N. (2014). The relationship between morphological and behavioral mimicry in hover flies (Diptera: Syrphidae). American Naturalist, 183(2), 281–289. https://doi.org/10.1086/674612/ASSET/IMAGES/LARGE/FG6.JPEG
Ramírez, F., & Davenport, T. L. (2013). Apple pollination: A review. Scientia Horticulturae, 162, 188–203. https://doi.org/10.1016/j.scienta.2013.08.007
The R Foundation. (2020). R: The R Project for Statistical Computing (4.0.3). https://www.r-project.org/
Thorsnæs, G. (2021). Hardanger. In Store norske leksikon. http://snl.no/Hardanger
van Steenis, J. (2016). The hoverfly (Diptera: Syrphidae) fauna of the nature reserve Hågadalen-Nåsten, Uppsala, Sweden. Entomologisk Tidskrift, 137, 111–129.
Weems, H. V. (2000). hover fly—Allograpta obliqua. Featued Creatures. https://entnemdept.ufl.edu/creatures/beneficial/hover_fly.htm
Willis, A. D. (2019). Rarefaction, Alpha Diversity, and Statistics. Frontiers in Microbiology, 10. https://www.frontiersin.org/articles/10.3389/fmicb.2019.02407
Wilson, J. S., Jahner, J. P., Williams, K. A., & Forister, M. L. (2013). Ecological and Evolutionary Processes Drive the Origin and Maintenance of Imperfect Mimicry. PLoS ONE, 8(4), e61610. https://doi.org/10.1371/journal.pone.0061610
Wotton, K. R., Gao, B., Menz, M. H. M., Morris, R. K. A., Ball, S. G., Lim, K. S., Reynolds, D. R., Hu, G., & Chapman, J. W. (2019). Mass Seasonal Migrations of Hoverflies Provide Extensive Pollination and Crop Protection Services. Current Biology, 29(13), 2167-2173.e5. https://doi.org/10.1016/j.cub.2019.05.036
About the author
If you had told me before I started my bachelor that I would be the crazy bug person, I would have laughed in your face. Hindsight is 20/20 and entomology has nevertheless become a subject near and dear in my heart. The work that goes into the taxonomy, the puzzle of finding out what species we´re dealing and constantly learning about new aspects of a family of insects is a fundamental part of why I enjoy what I enjoy. I chose to take BIO299 because I had a prior taste for species identification and wanted to further explore practical lab experience with a larger assignment. During my work in BIO299, I unexpectedly fell head-over-heels in love with the hoverfly family, and it has, and will likely remain my passion for quite some time.





1 Response
[…] de Miranda, S. (2023). The Hidden Pollinators of Inner Hardanger: a study in Hoverfly (Syrphidae) Diversity, Abundance, and…. Bikuben […]