The habitat uses and behaviour of Black Grouse (Lyrurus tetrix) in western Norwegian heathlands, in winter
Published · Updated

A report by Donna Bertrand, Arild Breistøl and Siri Haugum
Abstract
Human activities keep exploiting lands to the detriment of biodiversity and species that are declining, owing to this habitat loss. Hence, understanding what use a species makes of its habitat contributes to preserving the species by establishing conservation strategies. Black grouse has a huge distribution range, from the Atlantic coast and northern Eurasia to south-eastern Siberia. Therefore, the majority of their distribution area has large climatic variation between summer and winter. Most studies on black grouse winter behaviour describe inland populations, whilst the habitat use of coastal populations is poorly described. We studied the habitat uses and winter behaviour of a coastal population of black grouse (Lyrurus tetrix) during winter 2021-2022 in the heathlands of Lygra, a coastal island in western Norway. We quantified vocal territoriality and the use of heathlands as feeding grounds during winter. We find that black grouse are present in the heathlands on the island through winter to mark territory and to feed. We used an acoustic recorder and recorded two types of calls, hisses and coos, proving that this coastal population of black grouse vocally defends their territory in winter, in contrast to inland populations. Black grouse appear to be most active shortly before and up to two hours after sunrise. No vocal activity is found in the afternoon or before sunset. We notice a decrease in activity from December to January, which may be due to increased rainfall and wind. Black grouse keep singing during both positive and negative temperatures but tend to reduce activity at very low temperatures. Fecal analyses confirm that they feed on the heathland shrub Calluna vulgaris in winter, and we did not observe any changes in their diet over the winter period.
Introduction
According to the 2019 report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, three-quarters of the global land-based environment has been significantly altered by human actions (IPBES, 2019). This land-use change can be the conversion of land cover, changes in the management of the ecosystem or agro-ecosystem, or changes in the spatial configuration of the landscape (IPBES, 2019). Therefore, it damages, divides and wipes out habitats to meet human needs and preferences. As a result, land-use change is the main driver of biodiversity loss worldwide and even species extinction as they lose their habitats (IPBES, 2019). Hence, a crucial part of restoring, conserving and managing species is to understand the habitat use.
The black grouse (Lyrurus tetrix), (Linnaeus, 1758), is a bird whose habitat has been altered for decades now. The species is widely distributed globally: ranging from Britain and North Eurasia to China (Lawrence, 2004; Zhang et al. 2020). Studies of Wegge & Kastdalen (2008) and Ciach (2015) showed that black grouse avoids dense woodland. The northern population is rather associated with young forest (Swenson & Angelstam, 1993; Gregersen, 2009; Ciach, 2015) or forest edge habitat (Paloc, 2004; Wegge & Kastdalen, 2008; Kurhinen et al., 2009), but coastal populations are also found in open heathlands (Baines, 1994; Starling-Westerberg, 2001). The differences in habitat use and winter behaviour between the inland and coastal populations is poorly described, but there are reasons to expect that there are important differences between these populations, in terms of habitat use and winter behavior. We expect these differences to be especially clear in winter, because of the lower temperatures, more snow cover, and higher predator pressure in the inland compared to the coastal areas.
Black grouse territoriality involves competitive behaviour such as calls and physical confrontations (Brown & Orians 1970). Studies on territorial behaviour is focused on inland populations and are concentrated on activities performed in spring, during the mating season (Rintamäki et al. 1999) when seasonal cackle period doubtless concurs (Kruijt & Hogan, 1967). Females may also show territoriality during the breeding season (Angelstam et al. 1985). Yet, males can have territory defence behaviour in autumn (Rintamäki et al. 1999) and visit leks most of the year (Gregersen, 2014, in Eastern Norway). According to Gregersen (2014), males fight for a position in the flock each day to establish dominance hierarchies. Black grouses also have a largely herbivorous diet (Lawrence, 2004). The diet of inland populations of black grouse consists of leaves and buds of berries, rowans, alders, spruces, and some seeds (Starling-Westerberg, 2001; Darmangeat & Dupérat, 2004; Paloc, 2004) and needles from resinous trees and bilberries (Vaccinium myrtillus) (Wegge & Kastdalen, 2008; Selås, 2019). Specific descriptions of the coastal populations’ diets are lacking, but high availability of rather notorious shoots of Calluna vulgaris suggest that this is a relatively more important plant in the coastal populations’ winter diet compared to inland populations.
Therefore, the interest of our study was to know more about winter behavior and habitat use (feeding and territoriality) of a coastal population of black grouse in western Norway, focusing on their diet and calls. This would highlight differences with inland populations of black grouse. So, based on frequent observations of black grouse in heathland habitats during winter, we recorded sound at dawn and dusk through winter to explore if the males were expressing territorial calls in a coastal black grouse population. We also studied plant material in feces samples to quantify how important heathlands are for feeding and foraging through winter, and to determine if the diet varies from early to late winter.
Material & Methods
1. Black grouse
We studied a coastal population of black grouse (Lyrurus tetrix) in Norway from December 2021 to February 2022. The population size has not been quantified, although observations of 15 males simultaneously (artsobservasjoner.no) suggest a population size of at least 30 individuals (Ellison and Magnani 1985, Marti et al. 2016). Despite the species being ranked as ‘Least concern’ on both the IUCN and the Norwegian red list for species, the species is nowadays in decline, and has even gone extinct in some distributional border areas (Warren & Baines, 2002). The driving causes for the decline are intensified land-use, climate change, parasite infestation, and predation (Baines et al., 2000 ; Ciach, 2015 ; Jahren et al., 2016 ; Hambálková et al., 2021). Moreover, the reproductive success of the species has also decreased (Ciach, 2015; Jahren et al., 2016). According to Gregersen (2009), a decline in the Norwegian black grouse population has been observed since 1970, especially into the south and in the very north of the country.
Black grouse are most active in the early morning and spend the rest of the day, 94 percent on average, hiding (Marjakangas, 1992 ; Darmangeat & Dupérat, 2004). Black grouses show their territoriality with actual defence (attacks, chases) but also with calls and displays that keep rivals out (Brown & Orians 1970). As Angelstam (1985) described it, “the cackle-call is used as an identifying territorial act and as an aggressive call in actual defence”.
2. Study site
The study site is located at Lygra (Fig.1), an 2.5 km² large island in Alver municipality in western Norway. Most of the island is covered in agricultural land, ranging from semi-natural heathlands in the northwest to more intensive land-use in the central part, and afforestation in the southeast. Approximately 60 people live in the central part of the island. The heathland is about 0,5 km² and reaches up to 20 meters above sea-level. Vegetation is dominated by common heather - Calluna vulgaris. There are also bushes and shrubs. The heathland is grazed year-round by old norse sheep.
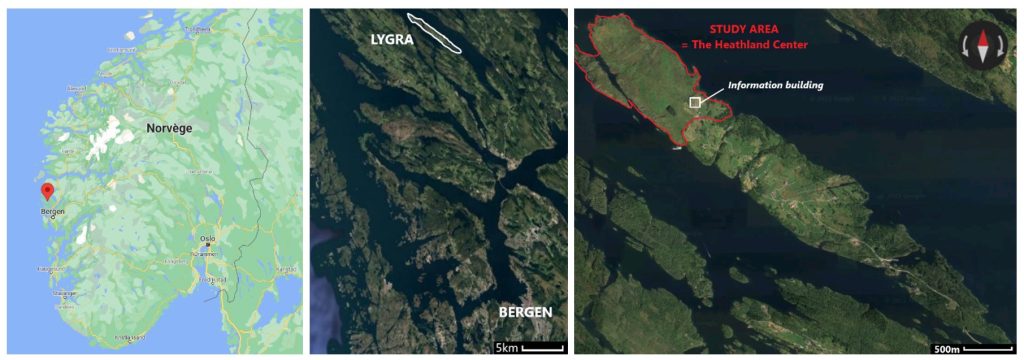
Figure 1. Location of Lygra, the island with heathlands outlined in red. Source : Google Maps, 2021. Location of Lygra. Google Maps.
Coastal heathlands are amongst the oldest cultural landscapes in Europe, reaching back 6000 years (Gjedrem & Log, 2020). Today, coastal heathlands have high conservation value throughout their range because of their biological diversity and cultural history. While the heathlands along the European Atlantic coasts are threatened by extinction - as much as 90 percent of European heathlands have been lost in the past 100 years due to cultivation, pollution, and overgrowth (Kaland & Kvamme, 2014) - authentic heathland coastal landscape of Lygra are well preserved, as it is a museum and research station used all year round. They are maintained by local farmers through periodical burning and continuous grazing. Once a year, prescribed burning is applied to parts of the heathland, creating a mosaic of fire patches with a fire return interval of approximately 25 years across the landscape (Fig. A1 in the appendix). This mosaic burning maintains fodder value of the vegetation and increases species richness through high microscale vegetation heterogeneity. The periodic burning and year-round grazing are not believed to affect black grouses directly, however this management is necessary to keep heathlands open and provide the habitat where black grouses can find food (heather, bilberry, buds, leaves) and display in open areas (Fig. A2 in the appendix).
3. Audio recordings
We used an acoustic recorder (Song Meter Mini from Wildlife Acoustics) to quantify territorial behaviour of black grouse in the heathland from December 2021 to the beginning of February 2022.
The recorder possesses an omni-directional microphone, has a recording bandwidth from 20Hz to 48kHz and can run 210 scheduled hours. These characteristics make it very suitable for bird recording. We placed it in a suppression in the terrain to shelter it from strong winds (Fig. 2), close to a hill with frequent observations of black grouses, in an early-successional stage of heathlands. The frequent visits to this hill were confirmed by the presence of fresh black grouse feces. The recorder is fixed high up on a wooden post so as not to be disturbed by the sheep. We programmed it to start recording from 7:00 to 11:00 and from 13:30 to 18:00, to capture the time just before and after sunrise and sunset. Every two or three weeks (dates in the appendix), we collected the recordings from the SD Card and changed the battery. Thus, this method is easy to implement, inexpensive and allowed us to study black grouses with little interference in their environment.

Figure 2. Location of the recorder in the heathlands. Source : Google Maps, 2021. Location of the recorder in the heathlands. Google Maps.
Then, to analyse the collected recordings, we used the version 2.3.3 of Audacity® recording and editing software [1]. It gives the spectrogram that enabled us to simply “look” for a sound through visualizing the audio recordings (method in Fig. A3 in the appendix). We knew the shape of a black grouse sound, thanks to literature (spectrograms in the study by Hambálková et al. (2021), Xeno-Canto website (spectrograms and audio recordings) and by recognizing the sound during our analyses. A spectrogram also provides complementary information like frequency, intensity and duration of the sound. Figure 3 illustrates an example of a spectrogram representing the audio recordings from two types of black grouse calls. Figure 3a shows a hissing call. This sound has a wider range of frequencies, often is shorter in duration and it is composed of two notes. Figure 3b illustrates a cooing call (Kruijt & Hogan, 1967). This sound has a smaller intensity, a lower and smaller frequency range, and may last longer because it is composed of several notes as it is a coo, a cackle. One repetition of each sound is visible.
[1] Audacity® software is copyright © 1999-2021 Audacity Team. The name Audacity® is a registered trademark.

Figure 3. Two sounds produced by a black grouse. A: hissing call. B: cooing call. The horizontal and vertical axes represent time and frequencies, respectively. The horizontal and vertical red arrows represent respectively the duration and the frequency range of the sound.
For all of the calls found on the spectrograms, we noted the date, the time of day and the time in the recording in which it occurs and specified the type of call. We counted and sorted them. To obtain the length of activity, we defined a period of activity as calls with less than one minute of silence in between. Then we calculated the sum of the periods of activity per day, during recording hours.
We also used weather data from the study period, collected by the bioCEED weather station at Lygra to answer if the activity is affected by temperature, precipitation or wind. First, we summed the amount of precipitation, per day, from 6:00 to 11:00, so it would correspond to the period where black grouse sing, and we looked for the maximal wind speed over the same time slot. Then, we calculated the average temperature, still between 6:00 and 11:00.
4. Faecal collection and analysis
In order to determine the diet of black grouses, to know if it changes during winter and to explore if black grouse also use the heathlands for foraging, we collected their feces. We collected it twice, around ten samples each time, at the end of November and in January to compare feces from the beginning and the end of winter. Each time, samples were collected (Fig. 4a) in two areas where black grouses are frequently observed during winter. Both areas are in the young successional vegetation stage of heathlands, meaning that there is less than 7 years since the last fire. One of the areas is a natural hill, whilst the other area has scientific installations with dimensions 3x3x1 meter (length x width x height) which black grouses have been observed to use for displaying. Then, in the lab, the samples were dried in the oven, weighed with a fine scale balance and placed in petri dishes before being fragmented with forceps and fingers. We looked for recognizable fragments of branches, seeds and leaves. We sorted them in smaller petri dishes (Fig. 4b) and weighted these portions. Thus, we calculated the ratio of their mass to the total mass of the sample and obtained the proportion of branches, seeds and leaves in the black grouse diet. But we also had to consider the part of the samples which remained unsorted, due to the tiny size of the fragments, to properly interpret the results. Indeed, the variations in identified sample proportions may have been caused by varying degrees of fragmentation of the plant matter in each sample. For that, we calculated the ratio between the mass of sorted matter and the total mass of the sample. Moreover, the results will be discussed as approximations and not as a direct measurement, because much of the leaves could have been digested already. When the grouse feeds on a plant, it eats a whole section of the shoot, which is a branch covered in leaves.
Results
Acoustic analysis
Spectrogram
Our acoustic analysis reveals the existence of black grouse calls in the heathlands in winter. We heard two types of calls (Fig. 5a) : cooing calls within a range of 900-2500 Hz (Fig. 5b) that often overlaps with background noise frequencies, and hissing calls (Fig. 5c), with wider signals in the frequency range of 1000-5000 Hz, sometimes up to even higher frequencies, depending on the intensity of the call. With these frequencies and the fact that they often are louder than coos, they are easier to filter out from background noise. We heard both isolated and grouped calls.
Timing of calls
Calls counting per time slot: Over the entire recording period, we never heard black grouse calls in the afternoon between 13:00 and 18:00. The results show that black grouses sing during our morning recording session, that is between 7:00 and 11:00. The peak of activity comes between 8:00 and 10:00 (Fig. 6), that is just before and after sunrise that happens between 8:30 and 9:30 from December to February. It also demonstrates that it is the same pattern for the two noises.
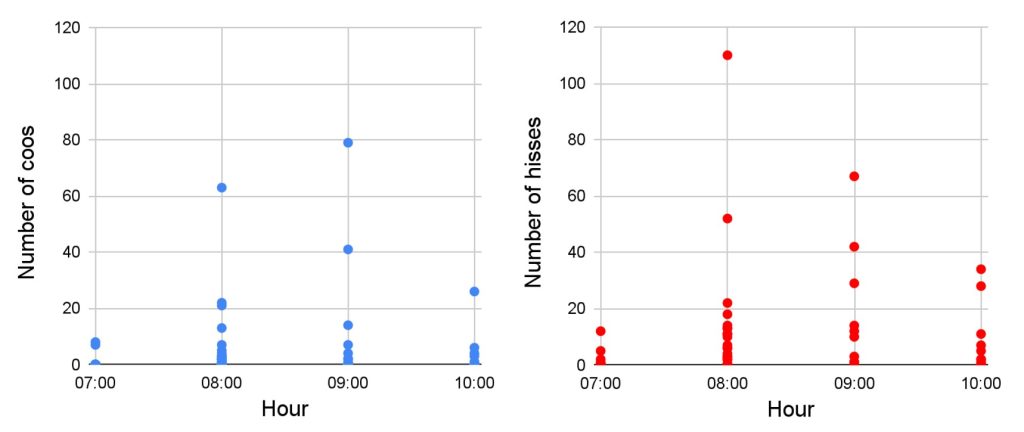
Figure 6. Number of coos and hisses recorded per day, according to the time slot. A dot represents one day.
Total number of calls and inactive days counting: We recorded 597 hisses and 362 coos, during our study period. We do not have continuous recordings due to the batteries running out that make us miss some days. In December, the maximum number of calls heard was 110 hisses and 79 coos against 12 hisses and 8 coos in January. No calls were heard during 6 days over 21 days of recordings in December and during 16 days over 20 days in January. That is, in December, 28 percent of the number of study days were without audio activity and 80 percent in January. Over the entire period of study, 43,5 percent of the days recorded audio activity. The overview of all the calls recorded is in Fig. A4 in the appendix.
Length of activity: By taking all the days of recording into account, the average length of activity is 179 seconds so almost 3 minutes (Fig. 7). By taking all the days where at least one song was heard (the “active days”), the average length of activity is 412 seconds (6 minutes and 52 seconds).
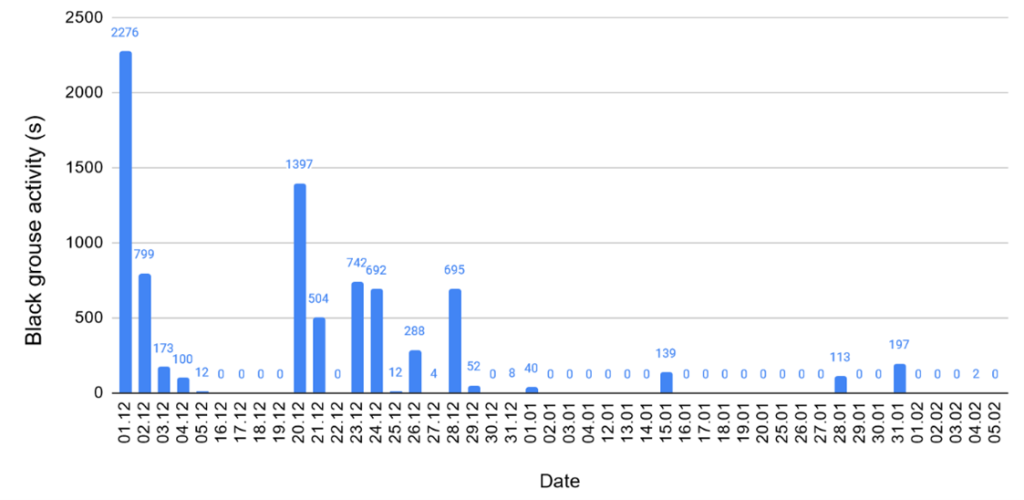
Figure 7. Length of black grouse activity in seconds per day. Some dates are missing due to data gaps in the recordings.
Weather data
By comparing the days with black grouse activity with the other days, we detect a trend: the length of activity of black grouse is more important when precipitation is low and the wind quiet (Fig. 8). For example, on December 1, there was apparently no rain, the wind was light and back grouse activity was over 2000 seconds, whereas during the first half of January, when the weather was rainy and windy, there was no black grouse activity. We however point out the absence of some data recordings from the weather station on January 29 between 10:00 and 11:00, on February 2 (all day) and on February 3, from 6:00 to 10:30. Thus, on Fig. 8, the weather values of these days may be higher because they are incomplete.
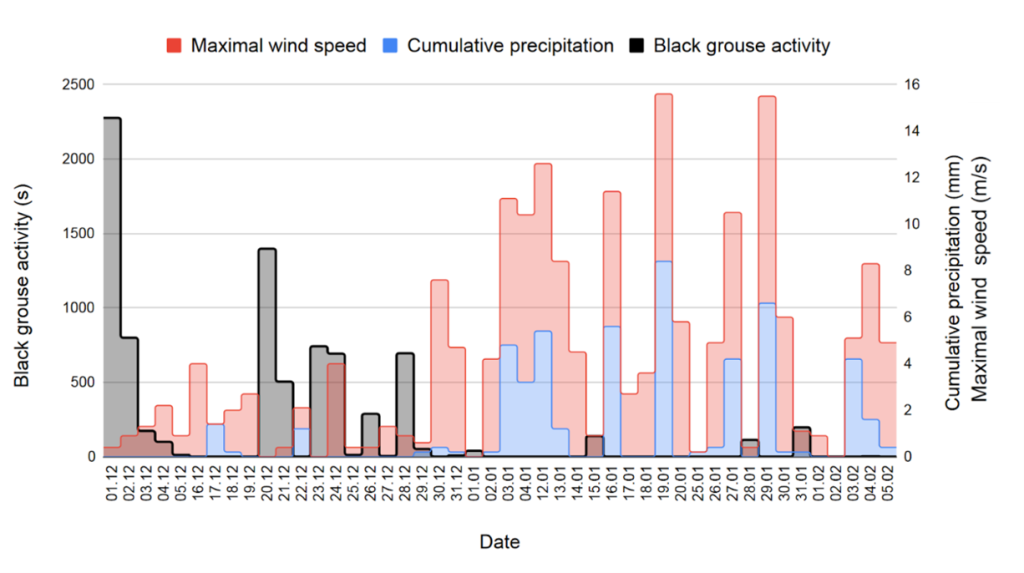
Figure 8. Length of black grouse activity and sum of precipitation and maximal wind speed per day. Precipitation and wind areas are stacked.
We find recorded calls during both positive and negative temperatures (Fig. 9). For example, on December 24, the average temperature was -1.8°C and black grouse activity was around 700 seconds. On January 15, the average temperature was 2.5°C and black grouse activity was around 200 seconds.
Diet linked to the surrounding vegetation based on collected feces in the heathlands
At first sight, fecal samples from November 30 and January 25 have a similar texture, color and when sorted, similar fragments. The composition consists of branches and leaves of Calluna vulgaris, and some seeds, for these two groups of collected feces. Branches often represent between 0 and 10% of the sample, except for sample 1 and 7 (Fig. 10a). But these values may be nuanced with Fig. 10b, which shows how much matter was sorted over the total amount of the sample. For example, sample 1 has the biggest amount of branches but because it has probably been more sorted, given that the ratio sorted matter/non-sorted matter is more important. Therefore, there is little difference between November and January and branches were more often found than leaves and seeds. We did not find any seed in the fecal samples from area 2, a hill with scientific installations.
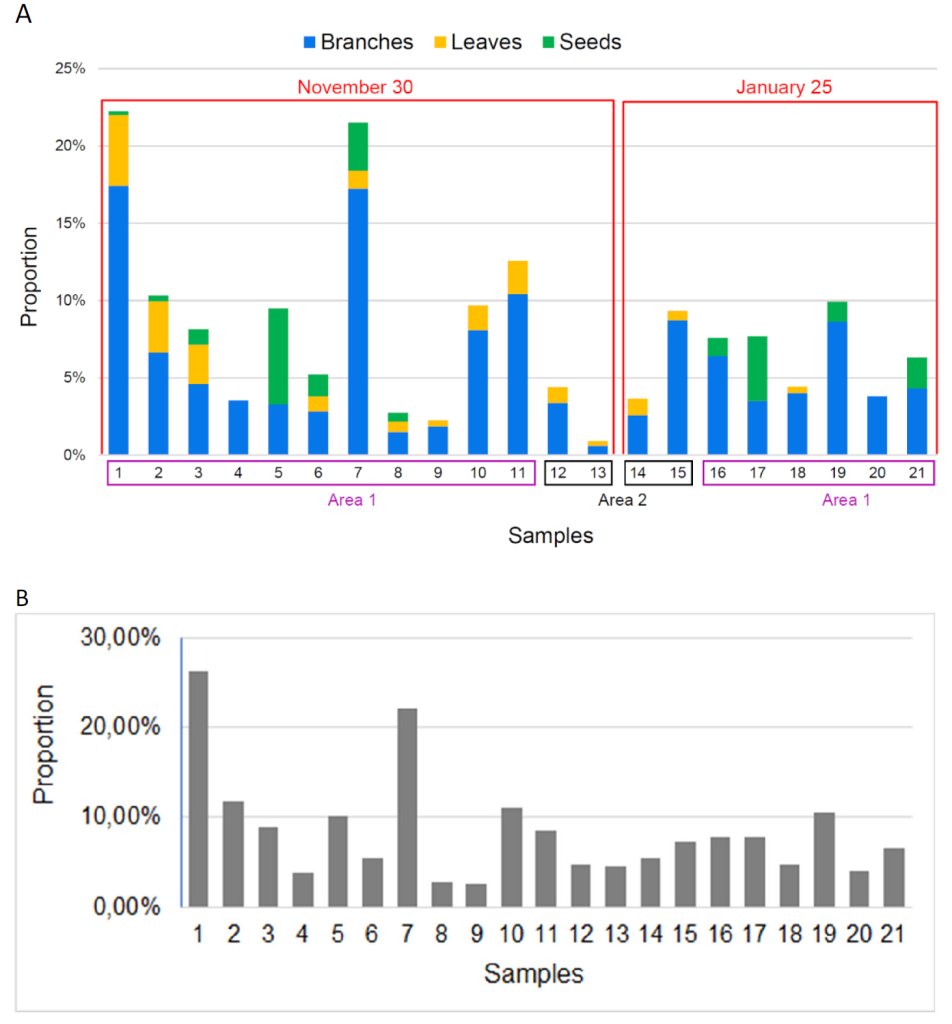
Figure 10. A: Proportion of branches, leaves and seeds in the sorted matter per fecal sample. B: Proportion of sorted matter per fecal sample.
Area 1 : Natural hill. Area 2 : Hill with scientific installation.
Discussion
Location of black grouse on Lygra
On Lygra, the coastal population of black grouse lives in the open and this is the first difference in habitat use with inland populations. In winter, the latter can dig tunnels in the snow (Darmangeat & Dupérat, 2004 ; Paloc, 2004) that allow them to rest by being sheltered from cold (Bocca et al. 2013) and predators such as, red fox, golden eagle, pine marten but mainly the northern goshawk in forest. These predators are common in forest/inland areas compared to coastal/open areas. However, there are some foxes at Lygra. Then, as black grouse hide in the bushes and shrubs on Lygra when they are not displaying or foraging, it is probably an anti-predator strategy, as it is a common strategy in many other birds.
According to Angelstam et al. (1985), cackle-calls could be located from about 600 m, or even from 1 km in open terrain according to Boback & Muller-Schwarze (1968). So, knowing the location of the recorder, we draw circles of 600 and 1000m radius (Fig. 11). They cover the study area and certify that the black grouse heard are found in the heathlands.

Figure 11. Distance from the recorder, represented by the red circles. Source: Google Maps, 2021. Distance from the recorder. Google Maps ].
Winter behaviour
Territoriality through calls
The literature of Norwegian black grouses is mainly based on inland populations who live in the forest all year. It is described that males have a strong territoriality in spring and cackle calls start three weeks before the beginning of incubation, late April (Angelstam et al. 1985), or that males and females show fighting behaviour and territory defence in autumn and in spring (Rintamäki et al. 1999). We heard hissing and cooing calls. Then, this study indicates that this coastal population of black grouse show territorial behavior during winter. Indeed, as mentioned before, territoriality is shown through calls that can be described as aggressive calls (Cramp, 1983). Especially hissing calls, that are frequently uttered during threatening, flutter-jumping and fighting (Kruijt & Hogan, 1967).
Our data match with the study of Hambálková et al. (2021), in which they found call frequencies from 352 to 4482 Hz for black grouse populations of Finland and Scotland. Yet, with our analysis, we can not exactly tell if black grouse calls frequencies fall below 900 Hz as it is mixed with background noises.
Winter activity
As for studies before (Angelstam et al. 1985; Kruijt & Hogan, 1967; Marjakangas, 1992), our results show that bird territorial activity is confined in the morning. We did not notice evening activity unlike Hjorth (1970) suggested. The daily total cackle period in winter is around three hours. That concurs with the study of Angelstam et al. (1985) and Marti and Pauli (1985).
Even though black grouse are out and singing in winter, they seem to be less active in January and February. The length of activity is below ten minutes and depends on the weather, especially precipitation and wind. On rainy and windy days, black grouse stay hidden and do not sing. Black grouse also tend to reduce their activity at low temperatures (Keller et al., 1979). According to the study of Marjakangas (1992), length of activity is correlated with ambient temperature but not with photoperiod.
Characteristics of the calls
The two types of calls that we were able to hear are defensive calls. Black grouse seem to use more often hissing calls over coos. Moreover, there is no hour difference between coos and hisses, both songs follow the same pattern.
Foraging and feeding
Black grouse inhabit the heathlands where they can find resources necessary to them. Studies often describe the winter diet of inland populations of black grouse, living in the forest where snow covers the floor (Bocca et al. 2013). Because of snow cover, these birds are forced to feed in trees and taller shrubs, so mountain pine needles or buds of many conifers are found in their diet (Bocca et al. 2013). But Lygra is not often covered by snow and the vegetation in the heathlands differs. The results of this study suggest that the common heather Calluna vulgaris make up most of the diet of the black grouse population at Lygra, as stated in the study of Baines (1994). During winter, their diet does not seem to change. Moreover, as Marjakangas (1992) deduced, if black grouse are only active in the morning, it is probably during this period that they feed. And because of the relatively low nutritive value and digestibility of their diet, they must feed regularly, because they do not accumulate substantial fat reserves (Bocca et al. 2013). This low digestibility explains that we were able to sort the samples and find recognizable fragments of branches, leaves and seeds.
Improvement ideas for our recording analysis
Margin of error: Even if the recorder is very good, strong wind and rain have been an issue for the acoustic analysis. It creates more background noises and even large signals and makes it more difficult to hear and see calls (Fig. A5 in the appendix). Therefore, one possibility is that we may have missed some calls during bad weather. The second possibility is that during bad weather, black grouse stay sheltered and do not sing anyway so we did not miss any call. Also, some cooing calls may be missing because, due to their low frequency range, they are barely visible and mix with background noises (Fig. A6 in the appendix). We also had to ensure to differentiate black grouse songs from other birds (Fig. A7 in the appendix).
Battery run-time and SD Card capacity: To avoid periods without any recording due to dead batteries or lack of space on the SD Card, we should have checked the recorder more often at the beginning of our study.
Recording hours: Some days, calls were recorded on the 10am recording, between 10:30 and 11:00 and even at 10:59. A quarter of the total days of study contain calls between 10:30 and 11:00. Therefore, it seems that black grouse can sing late in the morning and it can be interesting to add one or two more hours in the recording session, that is from 7 to 1pm. Moreover, we were not able to know how many black grouse we heard during the audio analyses.
Conclusion
On Lygra, the coastal population of black grouse uses the habitat for roosting, marking their territoriality and foraging. The heathlands are then vital for them whereas inland populations inhabit forests and clearings. These coastal black grouse keep their territorial behavior through calls, to a higher degree than inland populations. They show morning activity that decreases during winter and depends on the weather, especially precipitation, wind and temperature. They feed on branches and leaves of Calluna vulgaris and seeds, and their diet does not seem to change during the winter. Knowing this information, is important in terms of species management. To go even further in this approach, it would be interesting to correlate the presence of black grouse in Norway with the conservation of Norwegian semi-natural landscapes and ecosystem services.
How to cite this article
Bertrand, D., Breistøl, A. & Haugum S. (2022). The habitat uses and behaviour of Black Grouse (Lyrurus tetrix) in western Norwegian heathlands, in winter Bikuben 1.
References
Angelstam, P., Jaarola, M. & Nordh, N. 1985. Are female Black Grouse Tetrao tetrix territorial?. Ornis Fennica, 62: 124-129.
Baines, D. 1994. Seasonal differences in habitat selection by Black Grouse Tetrao tetrix in the northern Pennines, England. Ibis 136(1): 39–43. DOI: 10.1111/j.1474-919X.1994.tb08129.x
Baines, D., Blake, K. & Calladine, J. 2000. Reversing the Decline : a Review of some Black Grouse Conservation Projects in the United Kingdom. Cahier d’éthologie. 20: 217-234.
Boback, A. W. & Miiller-Schwarze, D. 1968. Das Birkhuhn. Die Neue Brehm-Biicherei . A. Ziemsen Verlag, Wittenberg Lutherstadt.
Bocca, M., Caprio, E., Chamberlain, D. & Rolando, A. 2013. The winter roosting and diet of Black Grouse Tetrao tetrix in the north-western Italian Alps. Journal of Ornithology, 155(1). DOI: 10.1007/s10336-013-1000-1
Brown, J.L., Orians, G.H. 1970. Spacing patterns in mobile animals. Annual Review of Ecology and Systematics 1: 239–262
Ciach, M. 2015. Rapid decline of an isolated population of the black grouse Tetrao tetrix: the crisis at the southern limit of the range. Eur J Wildl Res 61, 623–627 (2015). DOI : 10.1007/s10344-015-0923-7
Cramp, 1983. Handbook of the birds of Europe, the Middle East and North Africa: the birds of the Western Palearctic, Waders to Gulls. Vol. 3. Oxford: Oxford University Press.
Darmangeat, P. & Dupérat, M. 2004. Encyclopédie des oiseaux d’Europe. Editions Artemis, 165-166.
Ellison, L. N. and Magnani, Y. 1985. Eléments de dynamique de population du Tétras lyre (Tetrao tetrix) dans les Alpes françaises. — Gibier Faune Sauvage, Game Wildl. 2: 63–84.
Gjedrem, AM & Log, T. 2020. Study of Heathland Succession, Prescribed Burning, and Future Perspectives at Kringsjå, Norway. Land. 9(12):485. DOI : 10.3390/land9120485
Gregersen, F. & Gregersen, H. 2009. Ongoing population decline and range contraction in Norwegian forest grouse. Ornis Norvegica 32: 170−180. DOI: 10.15845/on.v32i0.163
Hambálková, L., Policht, R., Horák, J. & Hart, V. 2021. Acoustic individuality in the hissing calls of the male black grouse (Lyrurus tetrix). PeerJ, 9:18837. DOI: 10.7717/peerj.11837
Hjorth, I. 1970. Reproductive behavior in Tetraonidae, with special reference to males. Viltrevy (Swedish Wildlife), 7 (4): 183-596
IPBES, 2019. Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Editors : Brondizio, E., Settele, J., Díaz, S. and Ngo, H.T. IPBES secretariat, Bonn, Germany. 1148 pages. DOI : 10.5281/zenodo.3831673
Jahren, T., Storaas, T., Willebrand, T., Moa, P. & Hagen, B. 2016. Declining reproductive output in capercaillie and black grouse – 16 countries and 80 years. Animal Biology, 66 : 363-400. DOI : 10.1163/15707563-00002514
Johnsgard, P.A. 1983. The Grouse of the World. University Of Nebraska Press, London.
Kaland, P.E. & Kvamme, M. 2014. Coastal heathlands in Norway - Description of 23 reference areas. The Norwegian Environment Agency, University of Bergen and Lyngheisenteret, Report.
Keller, H., Pauli, H. & Glutz von Blotzheim, U. 1979. Feeding ecology in winter of the Black Grouse Tetrao tetrix in the Bernese Alps, Switzerland. Orn. Beob.76:9-32.
Kruijt, J.P., Hogan, J.A 1967. Social Behavior on the Lek in Black Grouse, Lyrurus tetrix tetrix (L.). Ardea, 55: 204-240. DOI : 10.5253/arde.v55.p203
Kurhinen, J., Danilov, P., Gromtsev, A., Helle, P. & Linden, H. 2009. Patterns of black grouse, Tetrao tetrix distribution in northwestern Russia at the turn of the millennium. Folia Zoologica, 58: 168-172.
Lawrence, S. 2004. The Encyclopedia of Animals : A complete visual guide. University of California Press.
Linnaeus, C. 1758. Systema naturæ per regna tria naturæ, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata, 1: 824.
Marjakangas, A. 1992. Winter activity patterns of the black grouse Tetrao tetrix. Ornis Fennica 69 :184-192.
Marti, C. A Bossert, HR Pauli. 2016. Bestand und Verbreitung von Birkhuhn Tetrao tetrix und Alpenschneehuhn Lagopus muta im Aletschgebiet von 1970 bis 2015. — Ornithol. Beob. 113: 1–30.
Marti, C. & Pauli, R. 1985. Wintergewicht, Masse und Altersbestimmung in einer alpinen Population des Birkhuhns Tetrao tetrix. Ornithologische Beobachtungen 82:231-241.
Paloc, R. 2004. Encyclopédie de la chasse. Editions Artemis, 73-76.
Rintamäki, P.T., Karvonen, E., Alatalo, R.V. & Lundberg, A. 1999. Why do Black Grouse males perform on lek sites outside the breeding season. Journal of Avian Biology 30:359−366.
Schoener, T. 1971. Theory of feeding strategies. Annual Review of Ecology and Systematics. 2 : 369-404 .
Selås, V. 2019. Annual change in forest grouse in southern Norway: variation explained by temperatures, bilberry seed production and the lunar nodal phase cycle. Wildlife Biology, 2019(1):1-9 (2019). DOI : 10.2981/wlb.00536
Starling-Westerberg, A. 2001. The habitat use and diet of Black Grouse Tetrao tetrix in the Pennine hills of northern England. Bird Study, 48:1 76-89. DOI: 10.1080/00063650109461205
Swenson, J. & Angelstam, P. 1993. Habitat separation by sympatric forest grouse in Fennoscandia in relation to boreal forest succession. Canadian Journal of Zoology 71: 1303−1310. DOI: 10.1139/z93-180
Warren, P. & Baines D. 2002. Dispersal, survival and causes of mortality in Black Grouse Tetrao tetrix in northern England. Wildlife Biology, 8:91-97. DOI: 10.2981/wlb.2002.013.
Wegge, P. & Kastdalen, Leif. 2008. Habitat and diet of young grouse broods: Resource partitioning between Capercaillie (Tetrao urogallus) and Black Grouse (Tetrao tetrix) in boreal forests. Journal of Ornithology, 149(2):237-244. DOI: 10.1007/s10336-007-0265-7
Zhang, C., Yang, L., Wu, S., Xia, W., Yang, L., Li, M., Chen, M., and Luan, X.. 2020. Use of historical data to improve conservation of the black grouse (Lyrurus tetrix) in Northeast China. Ecosphere 11( 3):e03090. 10.1002/ecs2.3090
About the author(s)
 My name is Donna Bertrand and I am a 22-years-old French student. I live in Brittany in western France. I entered Institut-Agro-Rennes, a French higher education institution of agronomics, to become an engineer in 3 years. After a first year of core curriculum, we have a 6-months-internship and for the last year, we must choose a specialization among 13 of them, before getting the engineering diploma. I am currently in the second year which is equivalent to a first year of a master’s degree. I was interested in two specializations: ethology and the political field that deals with environmental issues. In order to choose, I took advantage of the 6-months internship to learn more about these two fields. Thus, I went to Malta at the Ministry for Environment and then to Norway at the University of Bergen. Working in Bergen alongside Siri Haugum lived up to my expectations. It enabled me to carry out a real project, to study animal behavior under natural conditions, to work with others and to write a scientific paper. I find studies like this important because they help to preserve the fauna and flora, and to raise awareness of environmental issues. I will always remember this enriching opportunity even though I completely changed my mind and finally chose my specialization : Urban Green Space Engineering.
My name is Donna Bertrand and I am a 22-years-old French student. I live in Brittany in western France. I entered Institut-Agro-Rennes, a French higher education institution of agronomics, to become an engineer in 3 years. After a first year of core curriculum, we have a 6-months-internship and for the last year, we must choose a specialization among 13 of them, before getting the engineering diploma. I am currently in the second year which is equivalent to a first year of a master’s degree. I was interested in two specializations: ethology and the political field that deals with environmental issues. In order to choose, I took advantage of the 6-months internship to learn more about these two fields. Thus, I went to Malta at the Ministry for Environment and then to Norway at the University of Bergen. Working in Bergen alongside Siri Haugum lived up to my expectations. It enabled me to carry out a real project, to study animal behavior under natural conditions, to work with others and to write a scientific paper. I find studies like this important because they help to preserve the fauna and flora, and to raise awareness of environmental issues. I will always remember this enriching opportunity even though I completely changed my mind and finally chose my specialization : Urban Green Space Engineering.